|
Shenzhen HAD Trading Co., Ltd.
|
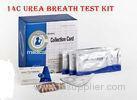
High accurate Test 13C Urea breath h pylori detection Test Medical Diagnostic Test kits
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
High accurate Test 13C Urea breath h pylori detection Test Medical Diagnostic Test kits
<s
High accurate Test 13C Urea breath h pylori detection Test Medical Diagnostic Test kits
Medical diagnostic reagents for h.pylori diagnosis test bacteria detection is applied for the in-
vitro diagnosis of Helicobacter pylori infection.
High accurate Medical Diagnostic Test kits for 13C Urea h pylori detection is applied for the in-vitro diagnosis of
Helicobacter pylori infection.
Helicobacter pylori is the primary cause of gastritis and peptic ulcer disease and has been linked to the onset of gastric
cancer in some individuals.
The need for a simple, non-invasive and accurate diagnostic technology for the detection of H. Pylori infection was met by
the development of urea breath test, which allows infection status to be evaluated without the need for costly and invasive
endoscopies.
Specification:
1. 13C-Urea capsule: 75mg/grain*20 grains/package
2. Breath bag: 40 pcs/package
3. Accurate Sensitivity >95%, specificity 95%~100%;
4. Analysis report within 5 minutes
5. Painless, Non-invasive, Safe;
The 13C-Urea Breath Test offers a range of advantages as below:
| Accurate | Sensitivity 95%, specificity 95%-100% |
| Comfortable | Non-invasive, no endoscopic tube insertion, well tolerated by patients. |
| Convenient | Near-patient testing, analysis made on site, minimal inconvenience for patients. |
| Swift | The entire test is finished in 30 minutes, test result available on site. |
| Simple | Easy operation, no special handling required. |
Who Should be Tested and Treated for H. Pylori?
7. Patient wishes

Advantages
Accurate:Sensitivity 95%, specificity 95%-100%.
Comfortable:Non-invasive, no endoscopic tube insertion, well tolerated by patients.
Convenient: Near-patient testing, analysis made on site, minimal inconvenience for patients.
Simple: Easy operation, no special handling required.
Swift: The entire test is finished in 30 minutes, test result available on site.
Company Information
Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd. was founded in 1996. We are the only High-Tech enterprise in China that owns the intellectual property rights to engage exclusively in the development and production of breath test kit and analysis equipment.
Our headquarters is located in National High-Tech Industrial Park in Shenzhen with a GMP certified workshop covering an area of over 2000m2. With a number of branches spreading over 33 provinces and municipalities in China, we have built up a comprehensive and efficient service network serving over 8000 hospitals across China.


Sample Room

Product Line